Actin Temporarily Shortens as It Slides Past Myosin

Hierarchical organization of skeletal musculus

Contractions of skeletal muscles allow vertebrate animals such as frogs to movement
Muscle contractions underlie motility
Muscle contraction is the activation of tension-generating sites within musculus cells.[1] [2] In physiology, muscle wrinkle does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such equally when holding a heavy book or a dumbbell at the aforementioned position.[i] The termination of muscle wrinkle is followed past musculus relaxation, which is a render of the musculus fibers to their low tension-generating country.[one]
Musculus contractions can be described based on two variables: length and tension.[1] A muscle contraction is described as isometric if the muscle tension changes merely the muscle length remains the aforementioned.[ane] [3] [4] [5] In contrast, a muscle contraction is isotonic if musculus tension remains the same throughout the wrinkle.[1] [3] [4] [five] If the musculus length shortens, the contraction is concentric;[1] [6] if the muscle length lengthens, the contraction is eccentric. In natural movements that underlie locomotor activity, muscle contractions are multifaceted as they are able to produce changes in length and tension in a fourth dimension-varying mode.[7] Therefore, neither length nor tension is likely to remain the same in muscles that contract during locomotor activeness.
In vertebrates, skeletal muscle contractions are neurogenic equally they require synaptic input from motor neurons. A single motor neuron is able to innervate multiple musculus fibers, thereby causing the fibers to contract at the same time. One time innervated, the poly peptide filaments within each skeletal musculus cobweb slide past each other to produce a contraction, which is explained by the sliding filament theory. The contraction produced can be described as a twitch, summation, or tetanus, depending on the frequency of activeness potentials. In skeletal muscles, musculus tension is at its greatest when the musculus is stretched to an intermediate length every bit described past the length-tension human relationship.
Unlike skeletal muscle, the contractions of smooth and cardiac muscles are myogenic (significant that they are initiated by the smooth or heart muscle cells themselves instead of beingness stimulated past an outside result such every bit nerve stimulation), although they can be modulated past stimuli from the autonomic nervous system. The mechanisms of contraction in these muscle tissues are like to those in skeletal musculus tissues.
Types [edit]

Types of muscle contractions
Muscle contractions can be described based on ii variables: force and length. Force itself tin be differentiated equally either tension or load. Muscle tension is the strength exerted by the muscle on an object whereas a load is the force exerted by an object on the musculus.[ane] When muscle tension changes without any corresponding changes in musculus length, the muscle contraction is described as isometric.[1] [3] [4] [v] If the muscle length changes while muscle tension remains the same, and then the muscle contraction is isotonic.[i] [3] [4] [5] In an isotonic wrinkle, the muscle length can either shorten to produce a concentric wrinkle or lengthen to produce an eccentric contraction.[one] [6] In natural movements that underlie locomotor action, musculus contractions are multifaceted as they are able to produce changes in length and tension in a time-varying fashion.[7] Therefore, neither length nor tension is likely to remain abiding when the muscle is active during locomotor activity.
Isometric contraction [edit]
An isometric contraction of a muscle generates tension without changing length.[1] [3] [4] [five] An case tin can be found when the muscles of the mitt and forearm grip an object; the joints of the paw do not move, but muscles generate sufficient force to prevent the object from being dropped.
Isotonic contraction [edit]
In isotonic contraction, the tension in the muscle remains abiding despite a change in musculus length.[1] [three] [4] [5] This occurs when a musculus's force of contraction matches the total load on the muscle.
Concentric contraction [edit]
In concentric contraction, musculus tension is sufficient to overcome the load, and the muscle shortens as it contracts.[8] This occurs when the force generated by the muscle exceeds the load opposing its contraction.
During a concentric contraction, a muscle is stimulated to contract according to the sliding filament theory. This occurs throughout the length of the musculus, generating a force at the origin and insertion, causing the muscle to shorten and changing the bending of the joint. In relation to the elbow, a concentric contraction of the biceps would cause the arm to bend at the elbow every bit the hand moved from the leg to the shoulder (a biceps curl). A concentric contraction of the triceps would alter the angle of the joint in the opposite direction, straightening the arm and moving the hand towards the leg.
Eccentric contraction [edit]
In eccentric contraction, the tension generated while isometric is bereft to overcome the external load on the musculus and the muscle fibers lengthen equally they contract.[9] Rather than working to pull a joint in the direction of the muscle contraction, the muscle acts to decelerate the joint at the end of a movement or otherwise control the repositioning of a load. This can occur involuntarily (e.g., when attempting to motion a weight likewise heavy for the muscle to lift) or voluntarily (e.g., when the muscle is 'smoothing out' a movement or resisting gravity such equally during downhill walking). Over the short-term, forcefulness training involving both eccentric and concentric contractions announced to increase muscular strength more than than grooming with concentric contractions alone.[ten] However, exercise-induced musculus harm is also greater during lengthening contractions.[11]
During an eccentric contraction of the biceps musculus, the elbow starts the movement while bent and and so straightens equally the hand moves away from the shoulder. During an eccentric contraction of the triceps muscle, the elbow starts the movement directly and then bends as the manus moves towards the shoulder. Desmin, titin, and other z-line proteins are involved in eccentric contractions, but their mechanism is poorly understood in comparing to crossbridge cycling in concentric contractions.[ix]
Though the muscle is doing a negative amount of mechanical piece of work, (work is beingness done on the musculus), chemical energy (originally of oxygen,[12] unlocked by fatty or glucose, and temporarily stored in ATP) is nevertheless consumed, although less than would be consumed during a concentric contraction of the same forcefulness. For case, ane expends more energy going upwardly a flight of stairs than going downwards the same flight.
Muscles undergoing heavy eccentric loading endure greater damage when overloaded (such as during muscle building or forcefulness training exercise) equally compared to concentric loading. When eccentric contractions are used in weight training, they are normally called negatives. During a concentric contraction, muscle myofilaments slide past each other, pulling the Z-lines together. During an eccentric contraction, the myofilaments slide by each other the contrary way, though the actual movement of the myosin heads during an eccentric wrinkle is non known. Practice featuring a heavy eccentric load tin can actually back up a greater weight (muscles are approximately 40% stronger during eccentric contractions than during concentric contractions) and also results in greater muscular damage and delayed onset muscle soreness ane to two days after training. Exercise that incorporates both eccentric and concentric muscular contractions (i.e., involving a strong contraction and a controlled lowering of the weight) tin produce greater gains in strength than concentric contractions alone.[ten] [13] While unaccustomed heavy eccentric contractions tin easily lead to overtraining, moderate training may confer protection against injury.[ten]
Eccentric contractions in motility [edit]
Eccentric contractions normally occur as a braking strength in opposition to a concentric contraction to protect joints from harm. During virtually any routine movement, eccentric contractions assistance in keeping motions smooth, just can also slow rapid movements such as a dial or throw. Role of grooming for rapid movements such every bit pitching during baseball game involves reducing eccentric braking allowing a greater power to be developed throughout the movement.
Eccentric contractions are being researched for their power to speed rehabilitation of weak or injured tendons. Achilles tendinitis[14] [15] and patellar tendonitis[16] (also known equally jumper's human knee or patellar tendonosis) have been shown to benefit from loftier-load eccentric contractions.
Vertebrates [edit]

In vertebrate animals, there are three types of musculus tissues: ane) skeletal, 2) shine, and 3) cardiac
In vertebrate animals, in that location are three types of musculus tissues: skeletal, smoothen, and cardiac. Skeletal muscle constitutes the majority of musculus mass in the body and is responsible for locomotor activity. Smooth muscle forms blood vessels, gastrointestinal tract, and other areas in the body that produce sustained contractions. Cardiac musculus make upwardly the centre, which pumps blood. Skeletal and cardiac muscles are called striated muscle because of their striped appearance under a microscope, which is due to the highly organized alternating blueprint of A bands and I bands.
Skeletal muscle [edit]

Organisation of skeletal muscle
Excluding reflexes, all skeletal muscles contractions occur every bit a result of signals originating in the brain. The brain sends electrochemical signals through the nervous system to the motor neuron that innervates several musculus fibers.[17] In the case of some reflexes, the signal to contract can originate in the spinal cord through a feedback loop with the grey matter. Other actions such as locomotion, breathing, and chewing have a reflex aspect to them: the contractions can be initiated both consciously or unconsciously.
Neuromuscular junction [edit]

Structure of neuromuscular junction.
A neuromuscular junction is a chemical synapse formed by the contact between a motor neuron and a musculus fiber.[18] It is the site in which a motor neuron transmits a point to a muscle fiber to initiate muscle wrinkle. The sequence of events that results in the depolarization of the musculus fiber at the neuromuscular junction begins when an action potential is initiated in the cell trunk of a motor neuron, which is and then propagated by saltatory conduction along its axon toward the neuromuscular junction. Once it reaches the last bouton, the action potential causes a Ca 2+
ion influx into the terminal by way of the voltage-gated calcium channels. The Ca 2+
influx causes synaptic vesicles containing the neurotransmitter acetylcholine to fuse with the plasma membrane, releasing acetylcholine into the synaptic cleft between the motor neuron final and the neuromuscular junction of the skeletal muscle fiber. Acetylcholine diffuses across the synapse and binds to and activates nicotinic acetylcholine receptors on the neuromuscular junction. Activation of the nicotinic receptor opens its intrinsic sodium/potassium channel, causing sodium to rush in and potassium to trickle out. Every bit a outcome, the sarcolemma reverses polarity and its voltage quickly jumps from the resting membrane potential of -90mV to every bit loftier equally +75mV as sodium enters. The membrane potential and then becomes hyperpolarized when potassium exits and is then adjusted back to the resting membrane potential. This rapid fluctuation is chosen the finish-plate potential[19] The voltage-gated ion channels of the sarcolemma adjacent to the stop plate open in response to the end plate potential. They are sodium and potassium specific and simply allow ane through. This wave of ion movements creates the activity potential that spreads from the motor end plate in all directions.[19] If activity potentials stop arriving, then acetylcholine ceases to exist released from the terminal bouton. The remaining acetylcholine in the synaptic cleft is either degraded by active acetylcholine esterase or reabsorbed by the synaptic knob and none is left to supplant the degraded acetylcholine.
Excitation–wrinkle coupling [edit]
Excitation–contraction coupling is the process by which a muscular activity potential in the musculus cobweb causes the myofibrils to contract.[20] In skeletal musculus, excitation–contraction coupling relies on a direct coupling between key proteins, the sarcoplasmic reticulum (SR) calcium release channel (identified equally the ryanodine receptor 1, RYR1) and voltage-gated L-type calcium channels (identified as dihydropyridine receptors, DHPRs). DHPRs are located on the sarcolemma (which includes the surface sarcolemma and the transverse tubules), while the RyRs reside across the SR membrane. The shut apposition of a transverse tubule and two SR regions containing RyRs is described as a triad and is predominantly where excitation–contraction coupling takes identify. Excitation–wrinkle coupling occurs when depolarization of skeletal muscle jail cell results in a muscle activeness potential, which spreads across the prison cell surface and into the muscle fiber's network of T-tubules, thereby depolarizing the inner portion of the muscle fiber. Depolarization of the inner portions activates dihydropyridine receptors in the terminal cisternae, which are in close proximity to ryanodine receptors in the adjacent sarcoplasmic reticulum. The activated dihydropyridine receptors physically interact with ryanodine receptors to activate them via foot processes (involving conformational changes that allosterically activates the ryanodine receptors). Every bit the ryanodine receptors open, Ca two+
is released from the sarcoplasmic reticulum into the local junctional space and diffuses into the bulk cytoplasm to cause a calcium spark. Note that the sarcoplasmic reticulum has a large calcium buffering capacity partially due to a calcium-binding poly peptide chosen calsequestrin. The nearly synchronous activation of thousands of calcium sparks by the activity potential causes a cell-wide increase in calcium giving ascension to the upstroke of the calcium transient. The Ca 2+
released into the cytosol binds to Troponin C by the actin filaments, to permit crossbridge cycling, producing strength and, in some situations, motion. The sarco/endoplasmic reticulum calcium-ATPase (SERCA) actively pumps Ca 2+
dorsum into the sarcoplasmic reticulum. As Ca 2+
declines back to resting levels, the force declines and relaxation occurs.
Sliding filament theory [edit]
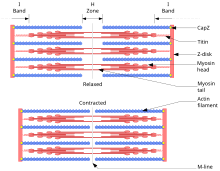
Sliding filament theory: A sarcomere in relaxed (in a higher place) and contracted (below) positions
The sliding filament theory describes a process used past muscles to contract. Information technology is a wheel of repetitive events that cause a thin filament to slide over a thick filament and generate tension in the muscle.[21] It was independently developed by Andrew Huxley and Rolf Niedergerke and past Hugh Huxley and Jean Hanson in 1954.[22] [23] Physiologically, this wrinkle is non uniform across the sarcomere; the central position of the thick filaments becomes unstable and can shift during contraction. However the deportment of rubberband proteins such equally titin are hypothesised to maintain uniform tension across the sarcomere and pull the thick filament into a cardinal position.[24]
Cross-bridge cycle [edit]
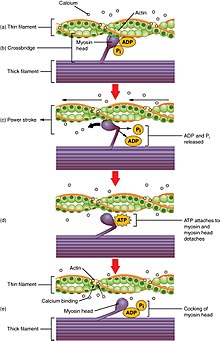
Cross-bridge cycling is a sequence of molecular events that underlies the sliding filament theory. A cross-bridge is a myosin projection, consisting of two myosin heads, that extends from the thick filaments.[1] Each myosin caput has ii binding sites: i for ATP and some other for actin. The binding of ATP to a myosin caput detaches myosin from actin, thereby allowing myosin to bind to another actin molecule. Once attached, the ATP is hydrolyzed past myosin, which uses the released free energy to motility into the "cocked position" whereby it binds weakly to a part of the actin bounden site. The remainder of the actin binding site is blocked by tropomyosin.[25] With the ATP hydrolyzed, the cocked myosin head now contains ADP + Pi. Ii Ca two+
ions demark to troponin C on the actin filaments. The troponin-Ca 2+
complex causes tropomyosin to slide over and unblock the remainder of the actin binding site. Unblocking the rest of the actin binding sites allows the two myosin heads to shut and myosin to bind strongly to actin.[25] The myosin head then releases the inorganic phosphate and initiates a power stroke, which generates a strength of ii pN. The power stroke moves the actin filament inward, thereby shortening the sarcomere. Myosin and so releases ADP but nonetheless remains tightly bound to actin. At the terminate of the power stroke, ADP is released from the myosin head, leaving myosin attached to actin in a rigor state until another ATP binds to myosin. A lack of ATP would result in the rigor land characteristic of rigor mortis. Once another ATP binds to myosin, the myosin head will again detach from actin and another crossbridges cycle occurs.
Cantankerous-bridge cycling is able to continue as long every bit there are sufficient amounts of ATP and Ca 2+
in the cytoplasm.[25] Termination of crossbridge cycling can occur when Ca ii+
is actively pumped back into the sarcoplasmic reticulum. When Ca 2+
is no longer present on the thin filament, the tropomyosin changes conformation back to its previous land so equally to block the binding sites again. The myosin ceases binding to the thin filament, and the muscle relaxes. The Ca ii+
ions leave the troponin molecule in order to maintain the Ca 2+
ion concentration in the sarcoplasm. The active pumping of Ca two+
ions into the sarcoplasmic reticulum creates a deficiency in the fluid around the myofibrils. This causes the removal of Ca 2+
ions from the troponin. Thus, the tropomyosin-troponin complex again covers the binding sites on the actin filaments and contraction ceases.
Gradation of skeletal muscle contractions [edit]
Twitch

Summation and tetanus
Three types of skeletal muscle contractions
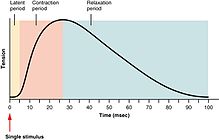
The forcefulness of skeletal muscle contractions can exist broadly separated into twitch, summation, and tetanus. A twitch is a single contraction and relaxation bicycle produced by an action potential inside the musculus fiber itself.[26] The time between a stimulus to the motor nervus and the subsequent contraction of the innervated muscle is called the latent period, which usually takes nearly ten ms and is acquired past the time taken for nerve action potential to propagate, the time for chemic manual at the neuromuscular junction, and so the subsequent steps in excitation-wrinkle coupling.[27]
If another muscle action potential were to be produced before the complete relaxation of a muscle twitch, so the next twitch will simply sum onto the previous twitch, thereby producing a summation. Summation can be achieved in two ways:[28] frequency summation and multiple fiber summation. In frequency summation, the force exerted by the skeletal muscle is controlled past varying the frequency at which action potentials are sent to musculus fibers. Activity potentials practice not arrive at muscles synchronously, and, during a contraction, some fraction of the fibers in the musculus will be firing at any given time. In a typical circumstance, when humans are exerting their muscles as hard equally they are consciously able, roughly ane-tertiary of the fibers in each of those muscles will burn at once[ citation needed ], though this ratio can be afflicted by various physiological and psychological factors (including Golgi tendon organs and Renshaw cells). This 'depression' level of wrinkle is a protective mechanism to foreclose avulsion of the tendon—the force generated by a 95% contraction of all fibers is sufficient to damage the body. In multiple cobweb summation, if the fundamental nervous arrangement sends a weak betoken to contract a muscle, the smaller motor units, being more excitable than the larger ones, are stimulated offset. Every bit the strength of the signal increases, more than motor units are excited in improver to larger ones, with the largest motor units having every bit much as 50 times the contractile strength as the smaller ones. As more and larger motor units are activated, the forcefulness of muscle contraction becomes progressively stronger. A concept known as the size principle, allows for a gradation of muscle force during weak contraction to occur in small steps, which then become progressively larger when greater amounts of strength are required.
Finally, if the frequency of musculus action potentials increases such that the muscle wrinkle reaches its peak force and plateaus at this level, then the contraction is a tetanus.
Length-tension relationship [edit]

Muscle length versus isometric force
Length-tension human relationship relates the forcefulness of an isometric contraction to the length of the muscle at which the contraction occurs. Muscles operate with greatest active tension when shut to an platonic length (often their resting length). When stretched or shortened beyond this (whether due to the action of the muscle itself or by an outside force), the maximum agile tension generated decreases.[29] This decrease is minimal for small deviations, but the tension drops off quickly as the length deviates further from the platonic. Due to the presence of rubberband proteins inside a musculus prison cell (such as titin) and extracellular matrix, as the muscle is stretched beyond a given length, there is an entirely passive tension, which opposes lengthening. Combined, there is a stiff resistance to lengthening an active muscle far beyond the superlative of active tension.
Strength-velocity relationships [edit]

Force–velocity relationship: correct of the vertical axis concentric contractions (the musculus is shortening), left of the axis eccentric contractions (the musculus is lengthened nether load); ability developed by the muscle in blood-red. Since power is equal to force times velocity, the musculus generates no power at either isometric force (due to cypher velocity) or maximal velocity (due to zero force). The optimal shortening velocity for ability generation is approximately 1-third of maximum shortening velocity.
Force–velocity relationship relates the speed at which a muscle changes its length (commonly regulated by external forces, such equally load or other muscles) to the amount of forcefulness that it generates. Force declines in a hyperbolic fashion relative to the isometric force as the shortening velocity increases, eventually reaching zero at some maximum velocity. The reverse holds truthful for when the muscle is stretched – force increases above isometric maximum, until finally reaching an accented maximum. This intrinsic belongings of active muscle tissue plays a function in the agile damping of joints that are actuated by simultaneously-agile opposing muscles. In such cases, the force-velocity contour enhances the force produced by the lengthening muscle at the expense of the shortening muscle. This favoring of whichever muscle returns the joint to equilibrium effectively increases the damping of the articulation. Moreover, the forcefulness of the damping increases with muscle strength. The motor system can thus actively command joint damping via the simultaneous wrinkle (co-contraction) of opposing muscle groups.[thirty]
Smooth muscle [edit]
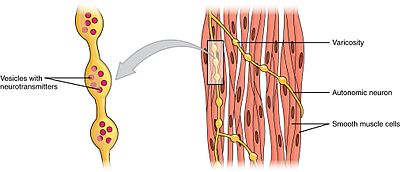
Swellings chosen varicosities belonging to an autonomic neuron innervate the smooth muscle cells.
Smooth muscles can exist divided into 2 subgroups: unmarried-unit of measurement and multiunit. Single-unit of measurement smooth musculus cells can be plant in the gut and blood vessels. Because these cells are linked together past gap junctions, they are able to contract as a functional syncytium. Unmarried-unit of measurement smooth muscle cells contract myogenically, which can be modulated by the autonomic nervous system.
Unlike single-unit smooth muscle cells, multiunit smooth musculus cells are institute in the musculus of the eye and in the base of hair follicles. Multiunit smoothen muscle cells contract past being separately stimulated by fretfulness of the autonomic nervous system. Every bit such, they permit for fine control and gradual responses, much like motor unit recruitment in skeletal muscle.
Mechanisms of smooth muscle contraction [edit]

Shine muscle contractions

Sliding filaments in contracted and uncontracted states
The contractile activeness of smooth muscle cells tin be tonic (sustained) or phasic (transient)[31] and is influenced by multiple inputs such as spontaneous electric activeness, neural and hormonal inputs, local changes in chemical composition, and stretch.[1] This is in dissimilarity to the contractile activity of skeletal muscle cells, which relies on a single neural input. Some types of smooth muscle cells are able to generate their own action potentials spontaneously, which ordinarily occur following a pacemaker potential or a slow wave potential. These activity potentials are generated by the influx of extracellular Ca 2+
, and not Na +
. Similar skeletal muscles, cytosolic Ca ii+
ions are also required for crossbridge cycling in smooth muscle cells.
The two sources for cytosolic Ca 2+
in shine musculus cells are the extracellular Ca 2+
entering through calcium channels and the Ca 2+
ions that are released from the sarcoplasmic reticulum. The elevation of cytosolic Ca 2+
results in more Ca 2+
binding to calmodulin, which and then binds and activates myosin light-chain kinase. The calcium-calmodulin-myosin calorie-free-concatenation kinase complex phosphorylates myosin on the twenty kilodalton (kDa) myosin low-cal bondage on amino acid residuum-serine nineteen, initiating contraction and activating the myosin ATPase. Unlike skeletal muscle cells, smooth muscle cells lack troponin, even though they contain the thin filament protein tropomyosin and other notable proteins – caldesmon and calponin. Thus, smooth muscle contractions are initiated by the Ca ii+
-activated phosphorylation of myosin rather than Ca ii+
binding to the troponin complex that regulates myosin binding sites on actin like in skeletal and cardiac muscles.
Termination of crossbridge cycling (and leaving the muscle in latch-state) occurs when myosin calorie-free chain phosphatase removes the phosphate groups from the myosin heads. Phosphorylation of the twenty kDa myosin low-cal chains correlates well with the shortening velocity of smooth muscle. During this period, there is a rapid flare-up of energy utilization as measured past oxygen consumption. Inside a few minutes of initiation, the calcium level markedly decreases, the twenty kDa myosin low-cal chains' phosphorylation decreases, and energy utilization decreases; however, force in tonic smooth muscle is maintained. During contraction of muscle, quickly cycling crossbridges course between activated actin and phosphorylated myosin, generating force. It is hypothesized that the maintenance of force results from dephosphorylated "latch-bridges" that slowly bike and maintain force. A number of kinases such as rho kinase, DAPK3, and protein kinase C are believed to participate in the sustained phase of contraction, and Ca 2+
flux may be significant.
Neuromodulation [edit]
Although smooth muscle contractions are myogenic, the rate and force of their contractions can exist modulated by the autonomic nervous system. Postganglionic nerve fibers of parasympathetic nervous organisation release the neurotransmitter acetylcholine, which binds to muscarinic acetylcholine receptors (mAChRs) on smooth muscle cells. These receptors are metabotropic, or M-protein coupled receptors that initiate a second messenger pour. Conversely, postganglionic nerve fibers of the sympathetic nervous system release the neurotransmitters epinephrine and norepinephrine, which demark to adrenergic receptors that are too metabotropic. The exact effects on the smooth muscle depend on the specific characteristics of the receptor activated—both parasympathetic input and sympathetic input can be either excitatory (contractile) or inhibitory (relaxing).
Cardiac muscle [edit]
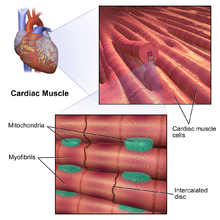
There are 2 types of cardiac muscle cells: autorhythmic and contractile. Autorhythmic cells practise not contract, but instead ready the pace of wrinkle for other cardiac muscle cells, which can exist modulated by the autonomic nervous organization. In dissimilarity, contractile muscle cells (cardiomyocytes) establish the majority of the heart musculus and are able to contract.
Excitation-contraction coupling [edit]
In both skeletal and cardiac muscle excitation-contraction (E-C) coupling, depolarization conduction and Ca2+ release processes occur. Even so, though the proteins involved are similar, they are singled-out in structure and regulation. The dihydropyridine receptors (DHPRs) are encoded by different genes, and the ryanodine receptors (RyRs) are distinct isoforms. Besides, DHPR contacts with RyR1 (chief RyR isoform in skeletal muscle) to regulate Ca2+ release in skeletal muscle, while the L-type calcium aqueduct (DHPR on cardiac myocytes) and RyR2 (primary RyR isoform in cardiac muscle) are not physically coupled in cardiac muscle, but face with each other by a junctional coupling.[32]
Dissimilar skeletal muscle, E-C coupling in cardiac muscle is thought to depend primarily on a mechanism called calcium-induced calcium release,[33] which is based on the junctional structure between T-tubule and sarcoplasmic reticulum. Junctophilin-2 (JPH2) is essential to maintain this structure, as well every bit the integrity of T-tubule.[34] [35] [36] Another protein, receptor accessory protein 5 (REEP5), functions to go on the normal morphology of junctional SR.[37] Defects of junctional coupling can result from deficiencies of either of the two proteins. During the procedure of calcium-induced calcium release, RyR2s are activated by a calcium trigger, which is brought near by the flow of Catwo+ through the Fifty-type calcium channels. Afterwards this, cardiac muscle tends to exhibit diad structures, rather than triads.
Excitation-wrinkle coupling in cardiac musculus cells occurs when an action potential is initiated past pacemaker cells in the sinoatrial node or Atrioventricular node and conducted to all cells in the heart via gap junctions. The action potential travels along the surface membrane into T-tubules (the latter are not seen in all cardiac jail cell types) and the depolarisation causes extracellular Ca 2+
to enter the cell via L-type calcium channels and possibly sodium-calcium exchanger (NCX) during the early part of the plateau stage. Although this Catwo+ influx simply count for about 10% of the Catwo+ needed for activation, it is relatively larger than that of skeletal muscle. This Ca 2+
influx causes a small local increase in intracellular Ca two+
. The increase of intracellular Ca 2+
is detected by RyR2 in the membrane of the sarcoplasmic reticulum, which releases Ca 2+
in a positive feedback physiological response. This positive feedback is known as calcium-induced calcium release[33] and gives rise to calcium sparks (Ca 2+
sparks[38]). The spatial and temporal summation of ~30,000 Ca ii+
sparks gives a cell-broad increment in cytoplasmic calcium concentration.[39] The increment in cytosolic calcium following the flow of calcium through the jail cell membrane and sarcoplasmic reticulum is moderated by calcium buffers, which bind a big proportion of intracellular calcium. As a result, a big increase in full calcium leads to a relatively small rise in complimentary Ca ii+
.[40]
The cytoplasmic calcium binds to Troponin C, moving the tropomyosin complex off the actin binding site allowing the myosin head to bind to the actin filament. From this bespeak on, the contractile machinery is essentially the same as for skeletal muscle (above). Briefly, using ATP hydrolysis, the myosin head pulls the actin filament toward the heart of the sarcomere.

Cardinal proteins involved in cardiac calcium cycling and excitation-contraction coupling
Following systole, intracellular calcium is taken upwardly by the sarco/endoplasmic reticulum ATPase (SERCA) pump dorsum into the sarcoplasmic reticulum set up for the side by side cycle to begin. Calcium is also ejected from the cell mainly by the sodium-calcium exchanger (NCX) and, to a lesser extent, a plasma membrane calcium ATPase. Some calcium is also taken up past the mitochondria.[41] An enzyme, phospholamban, serves every bit a restriction for SERCA. At low eye rates, phospholamban is active and slows down the activity of the ATPase so that Ca 2+
does not take to get out the prison cell entirely. At high heart rates, phospholamban is phosphorylated and deactivated thus taking most Ca ii+
from the cytoplasm back into the sarcoplasmic reticulum. One time once again, calcium buffers moderate this fall in Ca 2+
concentration, permitting a relatively small subtract in free Ca two+
concentration in response to a large modify in full calcium. The falling Ca 2+
concentration allows the troponin circuitous to dissociate from the actin filament thereby catastrophe contraction. The heart relaxes, allowing the ventricles to fill up with blood and begin the cardiac cycle again.
Invertebrates [edit]
Round and longitudinal muscles [edit]

A simplified image showing earthworm movement via peristalsis
In annelids such as earthworms and leeches, circular and longitudinal muscles cells form the body wall of these animals and are responsible for their movement.[42] In an earthworm that is moving through a soil, for case, contractions of circular and longitudinal muscles occur reciprocally while the coelomic fluid serves as a hydroskeleton by maintaining turgidity of the earthworm.[43] When the circular muscles in the inductive segments contract, the anterior portion of beast's body begins to constrict radially, which pushes the incompressible coelomic fluid forward and increasing the length of the animal. As a result, the front terminate of the animal moves forwards. Equally the front end terminate of the earthworm becomes anchored and the circular muscles in the anterior segments get relaxed, a moving ridge of longitudinal musculus contractions passes backwards, which pulls the residue of animal's trailing body forward.[42] [43] These alternating waves of circular and longitudinal contractions is called peristalsis, which underlies the creeping movement of earthworms.
Obliquely striated muscles [edit]
Invertebrates such equally annelids, mollusks, and nematodes, possess obliquely striated muscles, which comprise bands of thick and thin filaments that are arranged helically rather than transversely, similar in vertebrate skeletal or cardiac muscles.[44] In bivalves, the obliquely striated muscles can maintain tension over long periods without using too much free energy. Bivalves use these muscles to keep their shells closed.
Asynchronous muscles [edit]
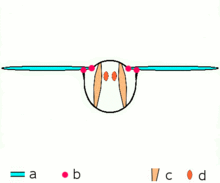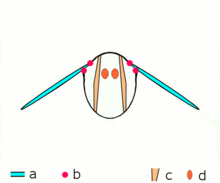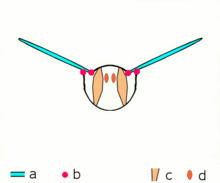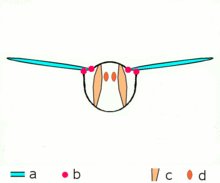
Asynchronous muscles ability flight in most insect species. a: Wings b: Wing articulation c: Dorsoventral muscles ability the upstroke d: Dorsolongitudinal muscles (DLM) power the downstroke. The DLMs are oriented out of the page.
Advanced insects such as wasps, flies, bees, and beetles possess asynchronous muscles that constitute the flying muscles in these animals.[44] These flight muscles are ofttimes chosen fibrillar muscles because they contain myofibrils that are thick and conspicuous.[45] A remarkable characteristic of these muscles is that they do not crave stimulation for each muscle contraction. Hence, they are chosen asynchronous muscles because the number of contractions in these muscles practice not correspond (or synchronize) with the number of activeness potentials. For example, a wing muscle of a tethered fly may receive activity potentials at a frequency of iii Hz but information technology is able to beat at a frequency of 120 Hz.[44] The high frequency beating is made possible considering the muscles are connected to a resonant system, which is driven to a natural frequency of vibration.
History [edit]

Electrodes touch a frog, and the legs twitch into the upwardly position[46]
In 1780, Luigi Galvani discovered that the muscles of dead frogs' legs twitched when struck by an electrical spark.[47] This was one of the kickoff forays into the study of bioelectricity, a field that still studies the electrical patterns and signals in tissues such as nerves and muscles.
In 1952, the term excitation–contraction coupling was coined to describe the physiological process of converting an electric stimulus to a mechanical response.[20] This process is fundamental to muscle physiology, whereby the electric stimulus is usually an action potential and the mechanical response is contraction. Excitation–wrinkle coupling tin be dysregulated in many diseases. Though excitation–wrinkle coupling has been known for over half a century, it is still an active area of biomedical inquiry. The general scheme is that an action potential arrives to depolarize the prison cell membrane. Past mechanisms specific to the muscle type, this depolarization results in an increase in cytosolic calcium that is chosen a calcium transient. This increase in calcium activates calcium-sensitive contractile proteins that and then utilise ATP to cause cell shortening.
The mechanism for musculus contraction evaded scientists for years and requires continued research and updating.[48] The sliding filament theory was independently developed past Andrew F. Huxley and Rolf Niedergerke and by Hugh Huxley and Jean Hanson. Their findings were published as two consecutive papers published in the 22 May 1954 result of Nature under the common theme "Structural Changes in Muscle During Contraction".[22] [23]
See also [edit]
- Anatomical terms of motility
- calcium-induced calcium release
- Cardiac activity potential
- Balk
- Dystonia
- Exercise physiology
- Fasciculation
- Hill'southward musculus model
- Hypnic jerk
- In vitro muscle testing
- Lombard's paradox
- Myoclonus
- Rigor mortis
- Spasm
- Uterine contraction
References [edit]
- ^ a b c d east f chiliad h i j thou l yard northward o Widmaier, Eric P.; Raff, Hersel; Strang, Kevin T. (2010). "Muscle". Vander's Human Physiology: The Mechanisms of Body Role (12th ed.). New York, NY: McGraw-Colina. pp. 250–291. ISBN978-0-321-98122-6.
- ^ Silverthorn, Dee Unglaub (2016). "Muscles". Human Physiology: An Integrated Approach (7th ed.). San Francisco, CA: Pearson. pp. 377–416. ISBN978-0-321-98122-6.
- ^ a b c d eastward f Aidley, David J. (1998). "Mechanics and energetics of muscular contraction". The Physiology of Excitable Cells (4th ed.). New York, NY: Cambridge University Press. pp. 323–335. ISBN978-0-521-57421-1.
- ^ a b c d due east f Sircar, Sabyasachi (2008). "Musculus elasticity". Principles of Medical Physiology (1st ed.). New York, NY: Thieme. p. 113. ISBN978-1-588-90572-7.
- ^ a b c d e f Bullock, John; Boyle, Joseph; Wang, Michael B. (2001). "Muscle contraction". NMS Physiology. Vol. 578 (4th ed.). Baltimore, Maryland: Lippincott Williams and Wilkins. pp. 37–56.
- ^ a b Kumar, Shrawan (2008). "Introduction and terminology". In Shrawan Kumar (ed.). Muscle strength (1st ed.). Boca Raton, FL: CRC Press. p. 113. ISBN978-0-415-36953-4.
- ^ a b Biewener, Andrew A. (2003). "Muscles and skeletons: The edifice blocks of creature motion". Beast Locomotion. Oxford Animal Biology Series. New York, NY: Oxford Academy Printing. pp. 15–45. ISBN978-0-198-50022-iii.
- ^ Faulkner JA (2003). "Terminology for contractions of muscles during shortening, while isometric, and during lengthening". Journal of Applied Physiology. 95 (2): 455–459. doi:x.1152/japplphysiol.00280.2003. PMID 12851415.
- ^ a b "Types of contractions". 2006-05-31. Retrieved 2007-ten-02 .
- ^ a b c Colliander EB, Tesch PA (1990). "Effects of eccentric and concentric musculus deportment in resistance grooming". Acta Physiol. Scand. 140 (1): 31–9. doi:10.1111/j.1748-1716.1990.tb08973.x. PMID 2275403.
- ^ Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA, Vrabas IS (2012). "Redox biological science of exercise: an integrative and comparative consideration of some overlooked problems". J. Exp. Biol. 215 (Pt 10): 1615–25. doi:10.1242/jeb.067470. PMID 22539728.
- ^ Schmidt-Rohr, Thou. (2020). "Oxygen Is the High-Energy Molecule Powering Circuitous Multicellular Life: Fundamental Corrections to Traditional Bioenergetics ACS Omega 5: 2221-2233. http://dx.doi.org/10.1021/acsomega.9b03352
- ^ Brooks, M.A; Fahey, T.D.; White, T.P. (1996). Exercise Physiology: Human Bioenergetics and Its Applications. (2nd ed.). Mayfield Publishing Co.
- ^ Alfredson, H; Pietilä, T; Jonsson, P; Lorentzon, R (1998). "Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis" (PDF). The American Journal of Sports Medicine. 26 (3): 360–6. doi:10.1177/03635465980260030301. PMID 9617396. S2CID 30259362.
- ^ Satyendra L, Byl Due north (2006). "Effectiveness of physical therapy for Achilles tendinopathy: An evidence based review of eccentric exercises". Isokinetics and Do Scientific discipline. 14 (1): 71–eighty. doi:10.3233/IES-2006-0223.
- ^ Cannell LJ, Taunton JE, Clement DB, Smith C, Khan KM (2001). "A randomised clinical trial of the efficacy of drop squats or leg extension/leg scroll exercises to treat clinically diagnosed jumper'south genu in athletes: airplane pilot written report". Br J Sports Med. 35 (1): 60–iv. doi:10.1136/bjsm.35.i.sixty. PMC1724276. PMID 11157465.
- ^ Tassinary; Cacioppo (2000). "The Skeletomotor organization: surface electromyography". In Cacioppo, John T.; Tassinary, Luois G.; Berntson, Gary Thousand. (eds.). Handbook of Psychophysiology (2d ed.). Cambridge: Cambridge University Printing. ISBN978-0-521-62634-7.
- ^ Levitan, Irwin; Kaczmarek, Leonard (August 19, 2015). "Intercellular communication". The Neuron: Cell and Molecular Biology (4th ed.). New York, NY: Oxford Academy Printing. pp. 153–328. ISBN978-0199773893.
- ^ a b Saladin, Kenneth S., Stephen J. Sullivan, and Christina A. Gan. Beefcake & Physiology: The Unity of Course and Function. 7th ed. New York: McGraw-Hill Education, 2015. Print.
- ^ a b Sandow A (1952). "Excitation-Wrinkle Coupling in Muscular Response". Yale J Biol Med. 25 (3): 176–201. PMC2599245. PMID 13015950.
- ^ Saladin, Kenneth (2012). Beefcake and Physiology: The Unity of Class and Function. New York: McGraw Hill. ISBN978-0-07-337825-1.
- ^ a b Huxley AF, Niedergerke R (1954). "Structural Changes in Muscle During Contraction: Interference Microscopy of Living Muscle Fibres". Nature. 173 (4412): 971–973. Bibcode:1954Natur.173..971H. doi:ten.1038/173971a0. PMID 13165697. S2CID 4275495.
- ^ a b Huxley H, Hanson J (1954). "Changes in the cross-striations of musculus during contraction and stretch and their structural interpretation". Nature. 173 (4412): 973–976. Bibcode:1954Natur.173..973H. doi:10.1038/173973a0. PMID 13165698. S2CID 4180166.
- ^ Horowits R, Podolsky RJ (November 1987). "The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the office of titin filaments". J. Jail cell Biol. 105 (5): 2217–23. doi:x.1083/jcb.105.5.2217. PMC2114850. PMID 3680378.
- ^ a b c Enoka, Roger M.; Pearson, Keir G. (2013). "The motor unit and muscle action". In Eric R. Kandel; James H. Schwartz; Thomas Thousand. Jessell; Steven A. Siegelbaum; A. J. Hudspeth (eds.). Principles of Neural Scientific discipline (fifth ed.). New York, NY: McGraw-Hill Medical. pp. 768–789. ISBN978-0-071-39011-8.
- ^ Feher, Joseph (2012). "Chapter 3.iv: Skeletal muscle mechanics". Quantitative Human Physiology: An Introduction. Academic Printing Serial in Biomedical Engineering (1st ed.). New York, NY: Academic Press. pp. 239–248. ISBN978-0-123-82163-8.
- ^ Khurana, Indu (2006). "Characteristics of muscle excitability and contractility". Textbook Of Medical Physiology (1st ed.). Elsevier. pp. 101–2.
- ^ Shwedyk, Due east.; Balasubramanian, R.; Scott, R. N. (1977). "A nonstationary model for the Electromyogram". IEEE Transactions on Biomedical Engineering. 24 (5): 417–424. doi:10.1109/TBME.1977.326175. PMID 892834. S2CID 1770255.
- ^ Gordon AM, Huxley AF, Julian FJ (1966). "The variation in isometric tension with sarcomere length in vertebrate musculus fibres". J. Physiol. 184 (ane): 170–92. doi:ten.1113/jphysiol.1966.sp007909. PMC1357553. PMID 5921536.
- ^ Heitmann, Stewart; Ferns, Norm; Breakpsear, Michael (2011). "Muscle co-wrinkle modulates damping and joint stability in a iii-link biomechanical limb". Frontiers in Neurorobotics. 5: five. doi:10.3389/fnbot.2011.00005. ISSN 1662-5218. PMC3257849. PMID 22275897.
- ^ Zhang, Y; Hermanson, ME; Eddinger, TJ (2013). "Tonic and phasic smooth muscle contraction is not regulated by the PKCα - CPI-17 pathway in swine stomach antrum and fundus". PLOS ONE. 8 (9): e74608. Bibcode:2013PLoSO...874608Z. doi:ten.1371/journal.pone.0074608. PMC3776813. PMID 24058600.
- ^ Martonosi, Anthony Due north.; Pikula, Slawomir (2003). "The network of calcium regulation in muscle". Acta Biochimica Polonica. 50 (1): 1–30. doi:ten.18388/abp.2003_3711. ISSN 0001-527X. PMID 12673344.
- ^ a b Fabiato, A. (1983). "Calcium-induced calcium release from the cardiac sarcoplasmic reticulum". American Periodical of Physiology. 245 (i): C1–14. doi:x.1152/ajpcell.1983.245.1.C1. PMID 6346892.
- ^ Guo, Ang; Zhang, Xiaoying; Iyer, Venkat Ramesh; Chen, Biyi; Zhang, Caimei; Kutschke, William J.; Weiss, Robert G.; Franzini-Armstrong, Clara; Song, Long-Sheng (2014-08-19). "Overexpression of junctophilin-2 does not enhance baseline role merely attenuates center failure development after cardiac stress". Proceedings of the National Academy of Sciences of the United States of America. 111 (33): 12240–12245. Bibcode:2014PNAS..11112240G. doi:x.1073/pnas.1412729111. ISSN 1091-6490. PMC4143026. PMID 25092313.
- ^ Wei, Sheng; Guo, Ang; Chen, Biyi; Kutschke, William; Xie, Yu-Ping; Zimmerman, Kathy; Weiss, Robert Thousand.; Anderson, Marker E.; Cheng, Heping; Vocal, Long-Sheng (2010-08-20). "T-tubule remodeling during transition from hypertrophy to heart failure". Circulation Research. 107 (4): 520–531. doi:ten.1161/CIRCRESAHA.109.212324. ISSN 1524-4571. PMC2927862. PMID 20576937.
- ^ Takeshima, H.; Komazaki, S.; Nishi, 1000.; Iino, M.; Kangawa, K. (July 2000). "Junctophilins: a novel family unit of junctional membrane complex proteins". Molecular Cell. half-dozen (1): 11–22. doi:ten.1016/s1097-2765(00)00003-4. ISSN 1097-2765. PMID 10949023.
- ^ Yao, Lei; Xie, Duanyang; Geng, Li; Shi, Dan; Huang, Jian; Wu, Yufei; Lv, Fei; Liang, Dandan; Li, Li; Liu, Yi; Li, Jun (3 Feb 2018). "REEP5 (Receptor Accompaniment Protein v) Acts every bit a Sarcoplasmic Reticulum Membrane Sculptor to Modulate Cardiac Office". Journal of the American Eye Association. 7 (iii). doi:10.1161/JAHA.117.007205. ISSN 2047-9980. PMC5850239. PMID 29431104.
- ^ Cheng H, Lederer WJ, Cannell MB (October 1993). "Calcium sparks: elementary events underlying excitation-contraction coupling in middle muscle". Science. 262 (5134): 740–4. Bibcode:1993Sci...262..740C. doi:ten.1126/science.8235594. PMID 8235594.
- ^ Cannell MB, Cheng H, Lederer WJ (November 1994). "Spatial not-uniformities in Ca 2+
i during excitation-contraction coupling in cardiac myocytes". Biophys. J. 67 (5): 1942–56. Bibcode:1994BpJ....67.1942C. doi:x.1016/S0006-3495(94)80677-0. PMC1225569. PMID 7858131. - ^ M., Bers, D. (2001). Excitation-contraction coupling and cardiac contractile force (2nd ed.). Dordrecht: Kluwer Bookish Publishers. ISBN9780792371571. OCLC 47659382.
- ^ Crespo LM, Grantham CJ, Cannell MB (June 1990). "Kinetics, stoichiometry and part of the Na-Ca exchange machinery in isolated cardiac myocytes". Nature. 345 (6276): 618–21. Bibcode:1990Natur.345..618C. doi:10.1038/345618a0. PMID 2348872. S2CID 4348240.
- ^ a b Hillis, David M.; Sadava, David E.; Toll, Mary Five. (2014). "Muscle and movement". Principles of Life (2nd ed.). Sunderland, MA: Sinauer Assembly. pp. 681–698. ISBN978-1-464-10947-8.
- ^ a b Gardner, C.R. (1976). "The neuronal control of locomotion in the earthworm". Biological Reviews of the Cambridge Philosophical Lodge. 51 (1): 25–52. doi:x.1111/j.1469-185X.1976.tb01119.x. PMID 766843. S2CID 9983649.
- ^ a b c Alexander, R. McNeill (2003). "Musculus, the motor". Principles of Animate being Locomotion (2nd ed.). Princeton, NJ: Princeton University Press. pp. 15–37. ISBN978-0-691-12634-0.
- ^ Josephson, R. K.; Malamud, J. K.; Stokes, D. R. (2000-09-15). "Asynchronous muscle: a primer". Journal of Experimental Biological science. 203 (eighteen): 2713–2722. doi:10.1242/jeb.203.18.2713. ISSN 0022-0949. PMID 10952872.
- ^ David Ames Wells, The science of common things: a familiar explanation of the first, 323 pages (page 290)
- ^ Whittaker, E. T. (1951), A History of the Theories of Aether and Electricity. Vol 1, Nelson, London
- ^ Huxley, H. East. (Apr 2000). "Past, Nowadays and Future Experiments on Musculus". Philosophical Transactions: Biological Sciences. 355 (1396): 539–543. doi:10.1098/rstb.2000.0595. JSTOR 3066716. PMC1692762. PMID 10836507.
Further reading [edit]
- Saladin, Kenneth Due south., Stephen J. Sullivan, and Christina A. Gan. (2015). Beefcake & Physiology: The Unity of Grade and Function. 7th ed. New York: McGraw-Colina Education.
- Krans, J. L. (2010) The Sliding Filament Theory of Muscle Contraction. Nature Education iii(9):66
External links [edit]
- Sliding Filament Model of Muscle Wrinkle
- Animation: Myofilament Wrinkle
Source: https://en.wikipedia.org/wiki/Muscle_contraction
0 Response to "Actin Temporarily Shortens as It Slides Past Myosin"
Postar um comentário